In a previous post we talked about water quality as it pertains to human consumption. However, water chemistry that is not harmful to humans can really be destructive to your plumbing system. One aspect of water chemistry is acid. First, a little refresher on high school chemistry.
What do the letters “pH” stand for….and why is the H capitalized? pH is an abbreviation for “power of hydrogen” where “p” is short for the German word for power, potenz and H is the element symbol for hydrogen. The H is capitalized because it is standard to capitalize element symbols. The abbreviation also works in French, with pouvoir hydrogen translating as “the power of hydrogen”.
pH is the measure of the acidity or the basicity of an aqueous solution. An aqueous solution is a solution in which the solvent is water. It is water with stuff dissolved in it. So, a cup of coffee with sugar is an aqueous solution. Solutions with a pH of less than 7 are said to be acidic, solutions with a pH greater than 7 are said to be basic or alkaline. Pure water has a pH of 7.
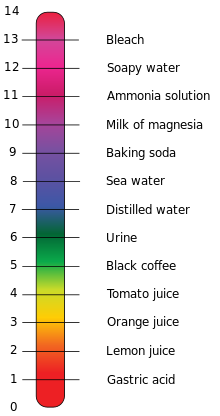
The pH scale is logarithmic. WHAT?! Yes, those mind numbing logarithm problems from high school algebra do have a real world application. The only thing I have to say about the acid logarithmic scale is that it is exponential and has a base of 10. So, tomato juice (pH of 4) is 10 times more acidic than Black coffee (pH of 5). If you want to know more about the pH scale and logarithms (in which case I’d question your sanity) you can look it up on Wikipedia. Or dust off your old Algebra 2 textbook.
Okay, so how does our well water become acidic? Remember the term “Acid Rain”? There you go-that’s how. When rain falls to the earth, carbon dioxide in the air is dissolved by the rain creating an aqueous solution of water and carbonic acid. This solution seeps into the ground, into aquifers, and eventually into your well. Limestone is a naturally occurring mineral that is able to eliminate acid from water. So if the ground your rain water passes through contains no limestone you will have very acidic water, a solid limestone layer under the ground will remove the acid and give you neutral water, and everywhere in between.
Acidic water is corrosive. That is to say, it causes pinholes in copper water piping, causes water heater and well water tanks to get leaks, prematurely deteriorates faucets, and generally destroys plumbing systems. You may notice blue-green stains on porcelain fixtures (toilets, sinks, etc…). This staining is copper from piping and fittings that has been dissolved into the water from the acidity of the water. Dissolving copper causes weakness in the piping eventually creating pinholes.
How do you raise your pH if you water doesn’t naturally pass through a layer of limestone? Read our next blog: How to Eliminate Acid in Your Home’s Water.
Same Day Emergency Service available! Call us at 410-840-8118 or fill in the form.





